POWERING AFFORDABLE CARE
with an efficient way to test for tuberculosis
According to the CDC, TB blood tests are preferred for:
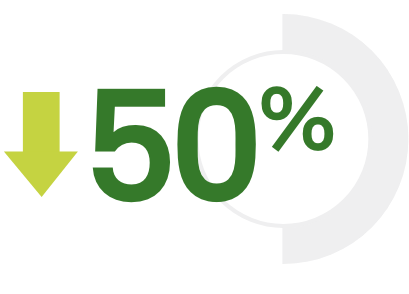
1 visit, 1 tube TB blood testing from Quest Diagnostics
There are 2 TB blood tests approved by the FDA: the T-SPOT®.TB test, and the QuantiFERON®-TB Gold Plus (QFT-Plus) test. Quest is the only lab that offers both—with results available from a single visit that can be reported directly to an EHR.

|
Easy specimen collection in 1 tube |

|
Approved for the immunocompromised patient as well as children ages 2+ |

|
Low false-positive rates compared to skin tests in BCG-vaccinated individuals5 |

|
Indeterminate/invalid rates <1% |

|
Up to 54-hour stability, strict ambientb |

|
Sensitivity was 95.6% [95% CI 91.6%–98.1%] in culture confirmed populations |

|
Specificity was 97.1% [95% CI 94.5%–98.7%] in a US low-risk population |

|
Flexible collection options: 1 tube or 4 tubes |

|
Innovative CD4+ and CD8+ T-cell response delivers a more comprehensive evaluation of a patient's immune response |

|
Low false-positive rates compared to skin tests in BCG-vaccinated individuals5 |

|
48-hour stability, refrigerated |

|
Sensitivity 94% |

|
Specificity 97% |